User:Mprosser17/sandbox
 | This is a user sandbox of Mprosser17. A user sandbox is a subpage of the user's user page. It serves as a testing spot and page development space for the user and is not an encyclopedia article. |
| Connexon | |
|---|---|
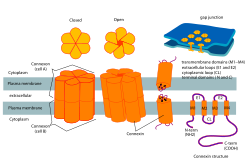 Connexon and connexin structure | |
| Details | |
| Identifiers | |
| Latin | connexona |
| Anatomical terminology | |
In biology, a connexon, also known as connexin hemichannels or pennexin channels, is an assembly of six proteins called connexins that form the pore for a gap junction between the cytoplasm of two adjacent cells. This channel allows for bidirectional flow of ions and signaling molecules.[1] The connexon is actually the hemichannel supplied by a cell on one side of the junction; two connexons from opposing cells come together to form the complete intercellular gap junction channel. However, in some cells, the hemichannel itself is active as a conduit between the cytoplasm and the extracellular space, allowing the transference of ions and small molecules lower than 1-2 KDa.
Connexons made of the same type of connexins are considered homomeric, while connexons made of differing types of connexins are heteromeric.[2]
Structure[edit]
Assembly[edit]
The assembly of connexons begins with synthesis of connexins within the cell and ends with the formation of gap junction channel plaques on the cell membrane. The connexin subunit proteins that make up connexons are synthesized on the membranes of the cells endoplasmic reticulum. These subunits are then oligomerized, or combined with other smaller parts, into connexons in the golgi apparatus.[3] The connexons are then delivered to their proper location on the plasma membrane. Connexons then dock with compatible connexons from the neighboring cell to form gap junction channel plaques.[3] A large part of this process is mediated by phosphorylation of different enzymes and proteins, allowing and preventing interaction between certain proteins.
General[edit]
Connexons contribute to the formation of gap junctions, and is an essential component of the electric synapses in neural pathways.[3] In a single gap junction, connexons will assemble around an aqueous porous membrane, forming a hemi-channel that is comprised of connexins. Connexins are the smaller protein molecules that make up connexons, that play a crucial part to the formation of gap junctions. On a structural basis, connexons are made up of 4 alpha helical transmembrane domains connected by 2 extracellular loops and 1 cytoplasmic loops, while both N and C terminals reside intracellularly. To further differentiate between connexon types by using their predicted molecular weight(ex: Connexon 43 is Cx 43 due to its molecular weight of 43 kDa) Connexons will form the gap junction by docking a hemi-channel to another hemi-channel in an adjacent cell membrane.[4] during this phase, the formation of an intercellular channels spanning both of the plasma membranes,occurs. Subsequently, this process leads to a better understanding of how electric synapses are facilitated between neurons.
Degradation[edit]
Connexon structure is degraded by its removal from the plasma membrane. Connexons will be internalized by the cell itself as a double membrane channel structure (due to the docking of hemi-channels). Now present in the cell membrane, connexons will be degraded by lysosomal pathways.
Cellular functions[edit]
Properties[edit]
The properties of the proteins that form the connexin channels determine the overall properties of the channels. The permeability and selectivity of the channels is determined by the width of the channels as well as molecular selectivity of connexins such as charge selectivity.[4] Channels are also voltage sensitive. The connexin channels have voltage-dependent gates that open or close depending on the difference in voltage between the interiors of the two cells[4]. Gates can also show voltage sensitivity depending on the difference in voltage from the interior and exterior of the cell (i.e. membrane potential).[4]
Modulation[edit]
Communication between gap-junctions can be modulated/regulated in many ways. The main types of modulation are:
- Chemical – one common type of chemical modulation is through the interaction of Ca2+ and certain domains of connexins. It is not completely understood, however, it is suggested that this interaction causes Ca2+ to block the pore of the channel. Another form of chemical modulation is through the response of the channel to acidification. It has been found that intracellular acidification causes a change in the C-terminal domain of connexins which then reduces the channel activity.[4]
- Protein Phosphorylation – protein phosphorylation regulates the communication between channels in multiple ways by controlling: connexin trafficking from the Golgi Apparatus, accumulation of connexons to certain areas, and degradation of unnecessary channels. The process of these actions is very complex but involvement of protein phosphorylation is known.[4]
- Humoral – humoral modulation of gap junction communication is done through many biomolecules such as neurotransmitters, growth factors, and various bioactive compounds. Neurotransmitters such as adrenaline and noradrenaline work in neuronal gap-junctions causing propagation of action potentials down neurons. These types of gap-junctions with this type of modulation are often found in neurons in cardiac tissue and vertebrate retina.[4]
Overall functions[edit]
Connexons play an imperative role in behavior and neurophysiology. There has not been much emphasis on their pathological function until recently and much of its function in detail is not fully understood yet. In the central nervous system (CNS) connexons play a major role in conditions such as epilepsy, ischemia, inflammation, and neurodegeneration.[1] The molecular mechanism as to how connexons play a role in the conditions listed above is not yet fully understood and is being further researched. Connexons also play an essential role in cell development; specifically their role in neurogenesis which deals with brain development as well as brain repair during certain diseases/pathology and assistance in both cell division as well as cell proliferation. The mechanism by which connexons aid in both of these processes is still being further researched however, it is currently understood that this mechanism involves purinergic signaling (form of extracellular signaling mediated by purine nucleotides and nueosides such as adenosine and ATP) and permeability to ATP.[1] Another important role of connexons is glucose sensing and signal transduction. Connexons cause changes in extracellular glucose concentrations effecting feeding/satiety behavior, sleep-wake cycles, and energy use.[1] Further studies indicate that there is an increase in glucose uptake mediated by connexons, whose mechanism is still not fully understood, under times of high stress and inflammation.[1] Recent research also indicates that connexons may also have an impact on synaptic plasticity, learning, memory, vision, and sensorimotor gating.
Related diseases[edit]
Some of the diseases associated with connexons is cardiovascular disease and a malfunction in the smaller units of connexons called connexions possibly leading to the onset of diabetes, the inability of the pancreas to produce insulin for glucose uptake by cells. The location where cardiovascular disease and diabetes type I and II can have an influence on is at the gap junctions where connexons facilitate rapid cell-to-cell interactions via electrical transmission at nerve endings such as in cardiac muscle and maintaining homeostasis in liver and kidney functions.The gap junction itself is a structure that is a specialized transmembrane protein formed by a connexon hemichannel.[5] In both cardiovascular disease and possibly in type II and I diabetes, both diseases are associated with the major protein connexin that makes up the gap junction.
In cardiovascular disease, Cx43 or connexin 43 a sudunit of the connexon strcucture is a general protein of the gap junction stimulating cardio myocyte muscle cells of the intercalated discs facilitating the synchronized beating of the heart. In the onset of cardiovascular disease the Cx43 subunit begins to show signs of oxidative stress, the ability of the heart to counteract the build up of harmful toxins due to age or diet that could possibly lead to reduced vascular functions.[5] Additionally reduced Cx43 expression in vascular tissue, which plays a part in ventricular remolding and healing of wounds after a myocardial infarction, are present in structural heart disease however the mechanisms of Cx43 in the heart are still poorly understood.[6] Overall, these changes in Cx43 expression and oxidant stress can lead to abnormalities in the coordinated beating of the heart predisposing it to cardiac arrhythmias .[5]
In diabetes, Connexons are correlated with type I and type II diabetes. Cx36 or connexin 36, part of the larger unit connexon, generates insulin excretion and glucose-induced insulin release from the gap junctions in the liver and pancreas.[2] Homeostasis in the liver and pancreatic organs is supported by an intricate system of cellular interactions by endocrine signaling operating short distances in the cellular membrane by way of signaling pathways utilizing ion channels, G-protein coupled receptors or tyrosine-kinase receptors, and by cell-to-cell contact.[2] The Gap junctions in these tissues arbitrate the intracellular signals between cells and the larger organ systems by connecting adjacent cells to each other in a tight fit so that concentration gradients are maintained and foreign substances do not get into the tissue and disrupt homeostasis. The purpose of the gap junction is to regulate the passage of ions, nutrients, metabolites, second messengers, and small biological molecules.[2] The subsequent loss of Cx36 substantially inhibits insulin production and intercellular signaling at the gap junction displaying the same types of symptoms in type II diabetes however; the function of Cx36 in type II diabetes in humans are unknown. Furthermore,Cx36 connexin is coded for by GJD2 gene, which has a predisposition on the gene locus for type II diabetes, and diabetic syndrome.[2]
References[edit]
- ^ a b c d e Cheung, Giselle; Chever, Oana; Rouach, Nathalie (2014-11-04). "Connexons and Pannexons: Newcomers in Neurophysiology". Frontiers in Cellular Neuroscience. 8: 348. doi:10.3389/fncel.2014.00348. PMC 4219455. PMID 25408635.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e Wright, Josephine; Richards, Toby; Becker, David (2012-03-01). "Connexins And Diabetes". Cardiology Research and Practice. 2012: 496904. doi:10.1155/2012/496904. PMC 3303578. PMID 22536530.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Thevenin, Anastasia F (2013-03-07). "Proteins and Mechanisms Regulating Gap-Junction Assembly, Internalization, and Degradation". Physiology. 28 (2): 93–116. doi:10.1152/physiol.00038.2012. PMC 3768091. PMID 23455769.
- ^ a b c d e f g Herve, Jean-Claude; Derangeon, Mickael (2012-09-01). "Gap-junction-mediated cell-to-cell communication". Cell and Tissue Research. 352 (1): 21–31. doi:10.1007/s00441-012-1485-6. PMID 22940728. S2CID 176666.
- ^ a b c Tomaselli, Gordon F. (2010-12-04). "Oxidant stress derails the cardiac connexon connection". Journal of Clinical Investigation. 120 (1): 87–89. doi:10.1172/JCI41780. PMC 2798705. PMID 20038808.
- ^ Zhang, Yan; Wang, Hongtao; Kovacs, Attila; Kanter, Evelyn; Yamada, Kathryn (2010-02-01). "Reduced expression of Cx43 attenuates ventricular remodeling after myocardial infarction via impaired TGF-β signaling". American Journal of Physiology - Heart and Circulatory Physiology. 298 (2): H477-87. doi:10.1152/ajpheart.00806.2009. PMC 2822575. PMID 19966054.
